Little Known Fact: PHCs & cVOCs Are Not The Only Contaminants You Can Detect In-Situ With HRSC Tooling
High-Resolution Site Characterization (HRSC) tooling, such as the MIP, LLMIP and Laser-Induced fluorescence (LIF) have been used countless times across Canada over the past ten years to delineate plumes of petroleum hydrocarbons (e.g. BTEX compounds, gasoline, diesel fuel, etc.) and chlorinated volatile organic compounds (e.g. PCE, TCE, TCA, VC, etc.) but what if those aren’t the contaminants of concern at your site? Is HRSC technology out of the question? As it turns out – perhaps not!
The Membrane Interface Probe (MIP) and Low Level Membrane Interface Probe (LLMIP) technologies provide large quantities of high resolution data on various in-situ conditions at a site. These features make the technology invaluable in gaining a more comprehensive understanding of the chemical and physical conditions in the subsurface.
Vertex’s MIP and LLMIP instrumentation is outfitted with three standard detectors onboard to respond and measure contaminants in-situ. These detectors: Flame Ionization Detector (FID), Photo-Ionization Detector (PID) and Halogen-Specific Detector (XSD), respond when specific compounds in vapour phase are swept across/into them. The signal/response that they produce is then plotted up versus depth and provided as discrete logs for each detector as in the example below from a PHC site.
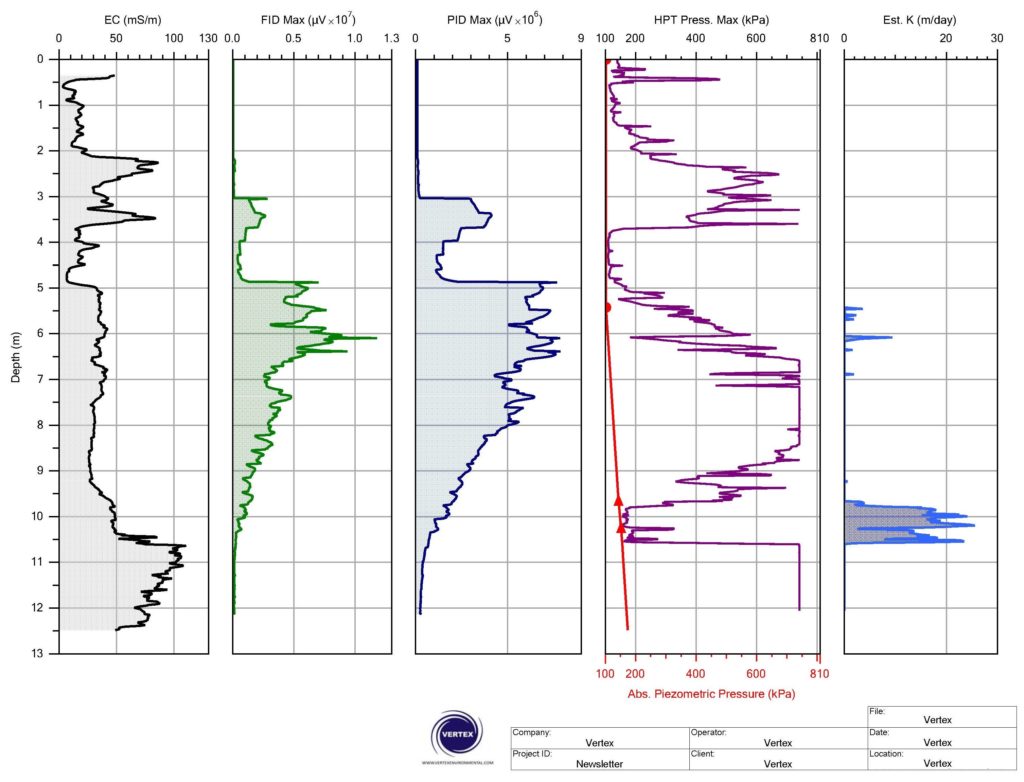
Figure 1: Example MiHpt Log with PHC impacts Responding in Unison on the FID and PID Detectors
However, clients often bring the more unusual and challenging sites to Vertex. Many have so called “non-standard” and emerging COCs such as PFAS compounds (PFOS & PFOA), ethylene glycol, sulfolane, bromacil, Freon 113 and others. We have often been asked by clients “What other compounds can be detected in-situ with your HRSC instruments?” Sometimes we already know: like for methane (yes), chloroform (yes) and even elemental mercury (yes – at an experimental level). Sometimes even we don’t know if our technology can detect specific COCs and then we complete bench-scale testing to find out. In fact, Vertex is currently completing in-house research into some historical soil sterilants (like bromacil and tebuthiuron) and their detectability with current and modified GC/detector alignments.
Bur for a more comprehensive list of parameters, Vertex recently reached out to Geoprobe® to determine what other compounds are detectable using the standard MIP and LLMIP configurations and detectors. You might find the list somewhat surprising!
| Chemical Compound: | Boiling Point (⁰C) | Molecular Weight (g/mol) | Density (g/mL) | Analytes Detected By: | |||
| PID* (10.6 eV Bulb) |
FID** | XSD | ECD*** | ||||
| 1,1,1,2-Tetrachloroethane | 130.5 | 167.8 | 1.553 | N | Y | Y | Y |
| 1,1,1-Trichloroethane | 74.0 | 133.4 | 1.320 | N | Y | Y | Y |
| 1,1,2,2-Tetrachloroethane | 146.5 | 167.9 | 1.250 | N | Y | Y | Y |
| 1,1,2-Trichloroethane | 115.0 | 133.4 | 1.435 | N | Y | Y | Y |
| 1,1-Dichloroethane | 57.2 | 96.7 | 1.213 | N | Y | Y | LS |
| 1,1-Dichloroethene | 32.0 | 97.0 | 1.213 | Y | Y | Y | LS |
| 1,2,4-Trichlorobenzene | 214.4 | 181.4 | 1.460 | Y | Y | Y | Y |
| 1,2,4-Trimethylbenzene | 170.0 | 120.2 | 0.876 | Y | Y | N | N |
| 1,2-Dibromoethane | 131.5 | 187.9 | 2.170 | Y | Y | Y | N |
| 1,2-Dichlorobenzene | 180.5 | 147.0 | 1.460 | Y | Y | Y | LS |
| 1,2-Dichloroethane | 83.7 | 99.0 | 1.253 | N | Y | Y | LS |
| 1,2-Dichloroethene | 60.0 | 97.0 | 1.280 | Y | Y | Y | LS |
| 1,2-Dichloropropane | 96.0 | 113.0 | 1.156 | N | Y | Y | Y |
| 1,3,5-Trimethylbenzene | 164.7 | 120.2 | 0.864 | Y | Y | N | N |
| 1,3,5-Trichlorobenzene | 208.0 | 181.4 | 1.140 | Y | Y | Y | Y |
| 1,3-Dichlorobenzene | 173.0 | 147.0 | 1.250 | Y | Y | Y | LS |
| 1,4-Dichlorobenzene | 174.0 | 147.0 | 1.300 | Y | Y | Y | LS |
| 1,4-Dioxane | 101.0 | 88.0 | 1.217 | LS | LS | N | N |
| 2-butanone (MEK) | 80.0 | 72.1 | 0.805 | Y | Y | N | N |
| 4-methyl-2-pentanone (MIBK) | 117.5 | 100.2 | 0.800 | Y | Y | N | N |
| Acetone | 56.5 | 58.1 | 0.790 | Y | Y | N | N |
| Benzene | 80.1 | 78.1 | 0.879 | Y | Y | N | N |
| Bromobenzene | 156.0 | 157.0 | 1.500 | Y | Y | LS | N |
| Bromochloromethane | 68.1 | 129.4 | 1.991 | N | Y | Y | N |
| Bromodichloromethane | 90.0 | 163.8 | 1.980 | N | Y | Y | LS |
| Bromoform | 149.0 | 252.7 | 1.590 | Y | Y | LS | N |
| Bromomethane | 3.6 | 94.9 | 3.974 | N | Y | Y | LS |
| Carbon tetrachloride | 76.7 | 153.8 | 1.809 | N | Y | Y | Y |
| Chlorobenzene | 131.0 | 112.6 | 1.110 | Y | Y | Y | Y |
| Chloroethane | 12.3 | 64.5 | 0.901 | N | Y | Y | LS |
| Chloroform | 61.0 | 119.4 | 1.480 | N | Y | Y | Y |
| Chlorotoluene | 162.0 | 126.6 | 1.072 | Y | Y | Y | N |
| cis-1,2-Dichloroethene | 60.3 | 97.0 | 1.280 | Y | Y | Y | LS |
| cis-1,3-Dichloropropene | 104.0 | 111.0 | 1.217 | Y | Y | Y | LS |
| Dibromochloromethane | 119.5 | 208.3 | 2.451 | N | Y | Y | N |
| Dibromomethane | 96.9 | 173.8 | 2.470 | Y | Y | Y | N |
| Diesel Range Organics (C10-C28) | 170-430 | Variable | Variable | Y | Y | N | N |
| Diisopropyl ether | 69.0 | 102.2 | 0.725 | Y | Y | N | N |
| Ethylbenzene | 136.0 | 106.2 | 0.860 | Y | Y | N | N |
| Freon 11 (Trichlorofluoromethane) | 23.8 | 137.4 | 1.494 | N | Y | Y | Y |
| Freon 113 (1,1,2-Trichloro-1,2,2-trifluoroethane) | 47.7 | 187.4 | 1.564 | N | Y | Y | Y |
| Freon 12 (Dichlorodifluoromethane) | -29.8 | 120.9 | 1.486 | N | Y | Y | LS |
| Gasoline Range Organics (C6-C10) | 60-220 | Variable | Variable | Y | Y | N | N |
| Isopropyl benzene (Cumene) | 152.0 | 120.2 | 0.862 | Y | Y | N | N |
| m, p-Xylene | 138.5 | 106.2 | 0.860 | Y | Y | N | N |
| Methane | -161.0 | 16.0 | 6.57×10-4 | N | Y | N | N |
| Methylene Chloride (Dichloromethane) | 40.0 | 84.9 | 1.327 | N | Y | Y | Y |
| Naphthalene | 218.0 | 128.2 | 1.145 | Y | Y | N | N |
| o-Xylene | 144.4 | 106.2 | 0.880 | Y | Y | N | N |
| Perfluorooctanoic acid (PFOA) | 190.0 | 414.0 | 1.800 | N | N | N | N |
| Perfluorrooctanesulfonic acid (PFOS) | 133.0 | 500.0 | 1.800 | N | N | N | N |
| Polychlorinated biphenyl (PCBs) | 340-375 | 291-361 | 1.18-1.57 | N | N | N | N |
| Styrene | 145.0 | 104.2 | 0.910 | Y | Y | N | N |
| tert butylmethyl ether (MTBE) | 55.2 | 88.2 | 0.740 | Y | Y | N | N |
| Tetrachloroethylene | 121.1 | 165.8 | 1.622 | Y | Y | Y | Y |
| Tetrahydrofuran | 66.0 | 72.1 | 0.889 | Y | Y | N | N |
| Toluene | 110.6 | 92.1 | 0.867 | Y | Y | N | N |
| trans-1,2-Dichloroethene | 47.5 | 97.0 | 1.260 | Y | Y | Y | LS |
| trans-1,3-Dichloropropene | 112.0 | 111.0 | 1.224 | Y | Y | Y | N |
| Trichloroethylene | 87.0 | 131.4 | 1.460 | Y | Y | Y | Y |
| Vinyl Chloride (Chloroethene) | -13.0 | 62.5 | 0.910 | Y | Y | Y | LS |
| Notes: | |||||||
| *Most common PID lamp electron voltage = 10.6eV. To respond on the PID, the analytes ionization potential must be below the lamp electron voltage. | |||||||
| **All compounds contain carbon and will burn in the FID if in high enough concentration | |||||||
| ***An ECD response is variable depending upon the number of chlorine atoms on a molecule and molecular structure. | |||||||
| LS = Low Sensitivity | |||||||
As it turns out, every parameter in the list above, with the regrettable exception of PCBs, PFOS and PFOA, can be detected by the MIP and LLMIP. That includes chlorobenzenes (tri-, di- and mono), 1,4-dioxane, Freons, MTBE and ketones like MEK and MIBK. More research into this technology is being conducted on an on-going basis and this list will undoubtedly expand. Similar testing is being completed on the other main HRSC instrument – the LIF and it has already been proven that it is not only limited to detecting mobile and residual PHC-based LNAPL above and below the water table.
Obviously, not all sites or contaminants are created equal so if your site has any of these compounds present and you are looking for a quick, efficient way to delineate the plume and move your Site toward closure be sure to consider a HRSC investigation using the MIP and LLMIP, or even the LIF.
Even if the contaminant at you site is not on the above list it doesn’t necessarily mean that an HRSC investigation is out of the question. Vertex would encourage you to send a sample of the COC or impacted media to us for FREE pre-screening testing in our in-house laboratory using our HRSC instrumentation. This would provide useful confirmation of COC detectability as well as a preliminary understanding of the possible detection limitations and contaminant response profiles on the detectors before even mobilizing to the site.
Then it may be possible to create detailed, interactive 3D visualizations of your uncommon COC plume at the site like the following example:
Figure 2: Example of a 3D Model of a cVOC Plume Generated from the MIP’s XSD Detector Response
The environmental industry is ever evolving with new, emerging and exotic contaminants cropping up every year. Not every site has the typical host of cVOC and PHC contaminants and it is important to understand what in-situ site characterization technologies are available to keep up with the changing needs of the environmental industry.

