Why ISCO is More Useful in 2016 Than Ever
People have been aware of the process of oxidation for millennia: ever since we learned to fashion tools and weapons from copper, iron and other metals.

Much later, once we started to understand the underlying chemistry, the word oxidation was used to describe reactions of various materials with oxygen to form oxides eg. iron oxide (rust). This chemical conversion is actually caused, at the atomic level, by electrons being stripped from the material undergoing oxidation. We now label all processes involving the loss of electrons as oxidation.
Historic and current day practices put the “power” of oxidation to practical use for a wide range of applications, including wastewater treatment processes. A related but relatively recent application is the use of oxidants for the destruction of environmental contaminants in soil and groundwater. In-situ Chemical Oxidation (ISCO) is the placement or injection of strong oxidants directly into the subsurface to react with and destroy contaminants in place.
ISCO is applicable to the degradation of a wide range of organic contaminants such as chlorinated solvents, petroleum hydrocarbons, polycyclic aromatic hydrocarbons and pesticides.
Early attempts at ISCO in the 1980s and 1990s made use of Fenton’s reagent (hydrogen peroxide catalyzed with iron). Typically Fenton’s was used for remediation of organic contaminants including petroleum compounds. By the late 1990s, permanganates were being employed in the field to address chlorinated solvent plumes. Later, persulphates gained favour as they are generally more stable and capable of degrading a wider range of contaminants. A fourth oxidant, ozone (gas), is a very powerful and broad acting oxidant. Although it has played a role in remediation efforts, ozone applications are limited due to practical and technical issues. Percarbonate is also used in ISCO applications.
The great advantage of ISCO technology is its ability to be deployed to treat otherwise difficult to manage contaminants. Examples include spills and leaks residing beneath utility corridors, within flowing sands, groundwater plumes and under buildings or at property boundaries. Excavating these impacts are often highly disruptive, costly, and sometimes impossible.
In spite of its many advantages, some property owners and environmental practitioners have felt the sting of a failed historical ISCO program. The leading causes of such failure were typically:
- Poor Site characterization, leading to poor Conceptual Site Models (CSM)
- Poor delivery and distribution of ISCO chemicals to allow contact with the contaminants
- Poor selection of appropriate ISCO chemistry
The good news is, after decades of experience and the continued evolution of ISCO technologies, in 2016 we are well equipped to address these former potential limitations head on.
For example, Site characterization tools such as Membrane Interface Probe (MIP) result in much better contaminant plume delineation and modelling, helping to address problems # 1 and #2 above.
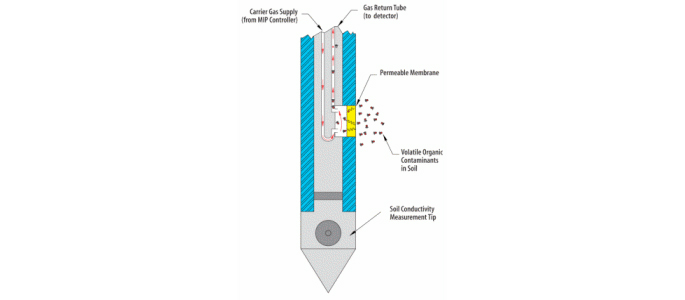
By inserting a probe into the ground, we can identify even narrow bands where contaminants may be “hiding”. ISCO agents can then be more effectively delivered to these discretely targeted zones.
Oxidant delivery itself is enhanced by advanced tools such as Direct Push Technologies (DPT). Hollow rods temporarily inserted into the ground to discrete depths enable solutions to be injected with very efficient vertical and lateral coverage and distribution across a Site.
A thorough understanding of contaminant reactivity and material compatibility is now established. The ISCO chemistry itself also continues to be advanced. Some recent examples:
- Safer activation chemistries with easier handling properties are currently being developed and in some cases are already on the market.
- Formulations combining oxidants (such as peroxide + ozone or permanganate + persulphate) often result in enhanced performance over the application of a single oxidant.
- Sustained release formulations and oxidant release canisters are now available to extend the reactivity period of reagents and also to mitigate contaminant migration across property boundaries. These sustained release formulations reduce Site mobilization charges and now enable ISCO to serve as a passive barrier technology.
- Carefully designed surfactants combined with ISCO reagents lead to improved desorption of soil contaminant mass into the aqueous phase for subsequent oxidation. Surfactants may be applied to contaminated soils in advance of or in tandem with ISCO reagents. In either case, the result is improved reactivity between oxidants and contaminants in the subsurface.
As in so many fields, combinations of technologies have now emerged to enhance the performance of ISCO: high resolution site characterization tools improve our ability to understand contaminant mass loadings and distribution; modern delivery techniques allow efficient and effective reagent application; and ever improving chemical formulations lead to safer, more efficient and economical treatment.

