Activated Carbon – What It Is and How It Works

Charcoal is produced by heating wood or other organic materials in the absence of oxygen at temperatures above 300⁰C. Early methods consisted of constructing earthen kilns over stacked wood. The wood is partially burned to generate heat.
Heating continues until only residual carbon and inorganic ash remain. The original vascular structure of the plant material and the pores created by escaping gases combine to produce a low density, highly porous material.
Historically, charcoal was a highly valued, readily transportable fuel source. Modern processes are more efficient at controlling air flow and heating rates, yielding greater quality carbon and introducing a variety of novel traits. Consequently, many industrial applications for carbon have also been discovered. For example, certain forms of charcoal have great utility in filtration.
Specific processes, including starting with a dense wood-like material, (e.g., coconut shells) leads to the formation of a charcoal with particularly fine pores (much higher surface area). This material is referred to as activated carbon (AC). One gram of AC has a surface area in excess of 3,000 m2!
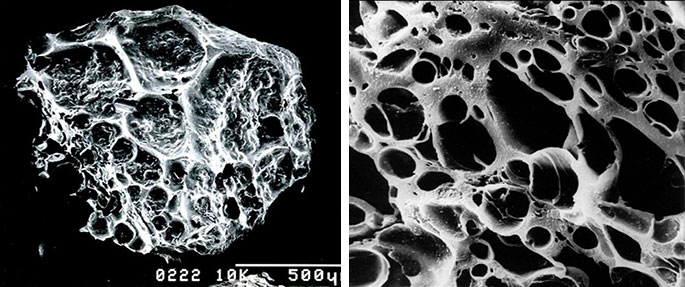
It is this high surface area that renders AC very useful as a filter and adsorbent of a wide range of chemical substances. When treating a waste stream above ground, contaminated media flows into the AC matrix and a “clean” effluent emerges. AC has long been trusted for purification of water, air, and waste streams, and is now effectively deployed for cleaning up groundwater in-situ.
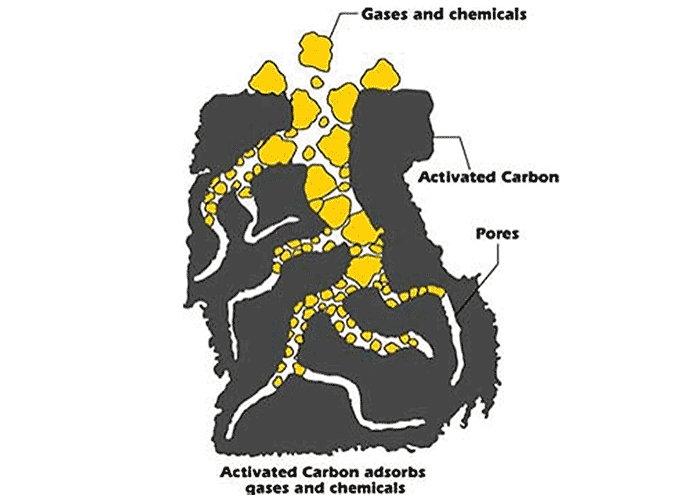
Relatively large pores provide for transport of contaminant molecules into the AC matrix. Smaller pores then “trap” or adsorb the contaminants. For above ground water filter applications, there is a limit or capacity to the adsorption. Once the micropores are filled, contaminant molecules will simply transport through the AC matrix via the larger pores (this is known as “breakthrough” of the AC). At this stage typically the carbon will require a “change out” to replace the spent filter media.
This industrial use of AC for water filtration has recently been adapted for the in-situ treatment of groundwater in the subsurface. The AC material is typically injected into the ground to intercept contaminated groundwater plumes. For groundwater remediation, change-out of carbon is an impractical option. Consequently, dosing with AC into the subsurface should account for the contaminant mass across the entire plume, in dissolved, sorbed, and free phases.
The more advanced forms of AC for groundwater remediation include additives formulated into the carbon that actively degrade contaminants upon contact. This serves to free up adsorption sites on the AC while ultimately eliminating the contaminant from the subsurface environment. Don’t use AC-based products that only provide containment, the subsequent treatment of the sorbed contaminants is critical to remedial success!
For examples of the use of an AC material to treat the subsurface check out this article. For examples of AC material case studies to treat cVOCs check out this article.

