The Birth of the Iron Wall
A permeable reactive barrier (PRB) is a permeable “wall” created below ground to clean up contaminated groundwater, it is a barrier to contaminants but allows the natural flow of groundwater to continue through the underground wall. According to one of the pioneers of PRB research, Dr. Robert Gillham of the University of Waterloo, the in-situ treatment of chlorinated solvents by a PRB was initially greeted with a high degree of suspicion and skepticism. His initial journal submission was actually rejected.
It has now been 20 years since the first commercial application of PRB technologies, and almost 30 years since PRB research began at UW. In honour of a technology with its roots in innovative Canadian-based research we present a two-part article on PRBs.
This month we share the story of how a serendipitous discovery led to the successful installation of hundreds of PRBs globally. We provide a brief history, explain the basic treatment processes and discuss the application of PRBs for the treatment of chlorinated solvents and other organic and inorganic contaminants.
In the second part of our article (next month), we will present design considerations, installation techniques and some little-known options for PRBs to target petroleum hydrocarbon (PHC) impacts.
Serendipity Strikes
During the 1980s, investigations were completed to determine whether PVC and other polymers were suitable candidates for use as groundwater monitoring well screens (anyone remember the days when only stainless steel screens were acceptable?). There was concern that adsorption of contaminants to the new PVC screens would cause a negative groundwater sampling bias. Surprisingly contaminant losses were observed when VOCs came into contact with certain metals. These losses were not consistent with an adsorption process, there was another process occurring that accelerated the VOC losses; Dr. Gillham was curious to know why. Further testing confirmed chlorinated solvents were actually degrading in the presence of many transitional metals, including iron. This realization resulted in the concept of placing iron in the subsurface to treat groundwater plumes.
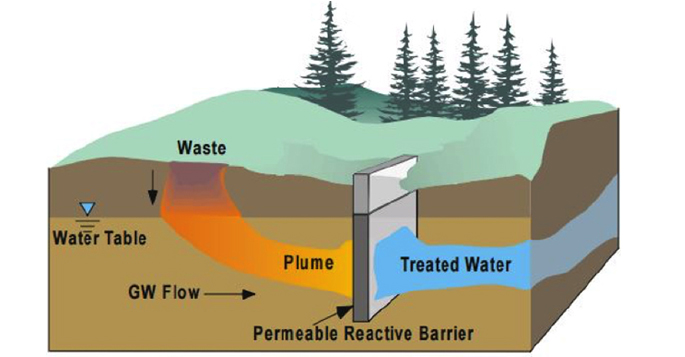
A PRB is an in situ treatment zone designed to intercept a groundwater plume. The “barrier” is designed to be more permeable than the surrounding aquifer media. The intent is to allow groundwater migration through the zone, but to retain or degrade contaminants, preventing their movement to downgradient areas.
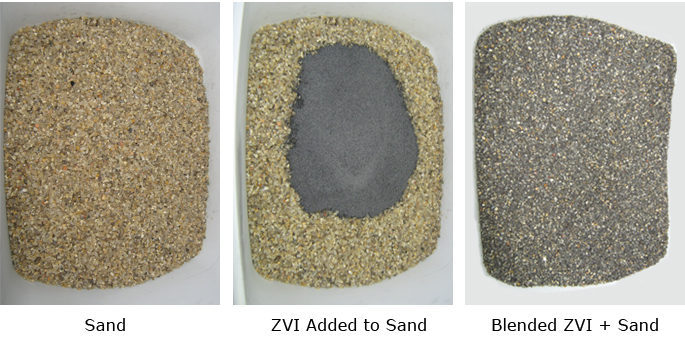
When environmental practitioners hear the term PRB, likely an “iron wall” most often comes to mind. This was one of the earliest and most common forms of PRB. Iron is a relatively inexpensive material. Typically iron filings are blended with sand and emplaced within a trench placed across the groundwater flow path. The most common form of PRB iron used is called zero-valent iron (ZVI).

After years of research by Dr. Gillham in the early 1990s, the first full-scale ZVI-based PRB was constructed in Sunnyvale, California in 1995. It was designed to replace a pump-and-treat system. This PRB demonstrated its ability to more cost-effectively, energy efficiently, and passively remediate a plume of TCE in groundwater. The pump-and-treat equipment was removed, resulting in significant operation and maintenance cost savings and allowing development of the above-ground area.
In natural systems, chlorinated solvents may degrade biologically via reductive dechlorination. Often the result is the accumulation of more toxic degradation products (i.e. vinyl chloride). ZVI degrades PCE and TCE abiotically (physical / chemical as opposed to biological processes) to non-toxic end products. The strong reducing conditions (lower ORP) created by ZVI allows for the degradation of contaminants without the accumulation of troublesome degradation products.
Alternative Processes
Processes for contaminant treatment within PRBs may be physical, chemical, or biological. Depending on the reactive material used within the PRB, groundwater contamination is removed through different processes, including: adsorption, complexation and precipitation (metals combine with other dissolved materials rendering them insoluble), reaction, and biodegradation. Within a ZVI PRB, microbial processes can be stimulated by adding carbon substrates and nutrients to enhance microbial activity.
A brief history of various PRB materials as they were first applied in the field is presented in the table below:
| Year First Used | Contaminant Treated | PRB Material |
| 1995 | TCE | Zero Valent Iron |
| 1995 | Nitrate | Solid Organic Amendments* |
| 1996 | Hexavalent Chromium (and TCE) | Zero Valent Iron |
| 1997 | Acid Mine Drainage | Solid Organic Amendments* |
| 1997 | Uranium | Phosphates (Apatite) |
| 1998 | Strontium-90 | Zeolites |
| 1999 | Perchlorate (and TCE) |
Solid Organic Amendments |
| 1999 | Phosphate | Iron and Steel Furnace Slag |
| 2002 | Arsenic | Solid Organic Amendments and Zero Valent Iron |
| 2005 | Creosote NAPL | Organophilic Clays |
*Solid Organic Amendments = wood chips, leafy compost
As you can see, there is more to PRBs than just “iron walls” and treating PCE and TCE.
Next month we will explore more recent developments in PRB materials, including hybrid formulations (combining iron and organic substrates) and application of PRBs to manage petroleum hydrocarbon contaminants.

